PESTS AND DISEASES OF FORESTRY IN NEW ZEALAND
Fighting Phytophthora in forests
Nari Williams and Michelle Harnett, New Zealand Tree Grower November 2017.
Scary-looking on paper and even scarier in forests, the word Phytophthora does not seem to have enough vowels in it. And how do you get your mouth around it? It comes from the Greek for plant ‘phyton’ and destruction ‘phthora’ and pronounced figh-toff-thor-a.
Hard to say or not, its plant-destroying nature means we have to talk about it. Pathogens from the Phytophthora genus are responsible for tree deaths around the world including in New Zealand where tree diseases have been in the news this past winter.
Outbreaks of red needle cast (RNC) have been reported around New Zealand. People watching the foliage of their pine trees turning reddish brown have asked if their trees were dying. Kauri dieback has also been reported to be spreading in the Waitakere Ranges. Wet conditions are causing tree decline and revealing a wider spread of the disease than previously recognised.
Both RNC and kauri dieback are caused by Phytophthora pathogens. Phytophthora are not quite fungi and not quite algae, but they have the characteristics of both. Technically they are omycetes.They are spread by two types of spores − active spores or zoospores which ‘swim’ in water to find new hosts, and passive spores that survive harsh conditions. When the spores
germinate they penetrate the foliage or roots of a host plant producing a network of fine threads or mycelia which produce spore-forming bodies called sporangia. Mature sporangia release new spores to start the cycle over again. Scarily, in wet and warm weather conditions, each cycle can be completed in a few days.
A new radiata pine disease
A mystery needle disease was found on the East Cape of the North Island during routine forest surveillance in 2008. Dark bands, or lesions, were noted on green needles, which turned red and were cast, giving rise to the local name of red needle cast. Successive waves of disease across winter left affected trees significantly defoliated through spring, the prime period of tree growth until the new flush established in summer.

Scion forest pathologists analysing affected needles found an unknown Phytophthora. In 2012, DNA sequencing identified it as Phytophthora pluvialis, a newly described species from Oregon. From Latin this time, pluvial means relating to rain.
The presence of a new pathogen affecting radiata pine, the backbone of the New Zealand forestry industry, caused great consternation. No one knew how far the disease would spread, how it would behave in following years and how it would affect forest productivity. With Phytophthora’s reputation as plant killers the worst case scenario of tree death was considered a real possibility.
Another very real threat was the possibility that the presence of the pathogen on logs would affect log exports. Research was put in place to understand if there was a link between this new species and RNC and to study if the pathogen was likely to be transported and establish in new areas domestically and overseas. Scion pathologists quickly established that the pathogen was only found in needles and not branches or stems. There was also the concern that viable spores could piggyback on the outside of logs. However, the majority of log exports from New Zealand are essentially subjected to a ‘temperature treatment’ as they pass through the equator on their way to northern hemisphere destinations. Logs on ships are exposed to temperatures greater than 35°C and hitch-hiking spores were found to be deactivated at much lower temperatures, so the log export trade was able to continue.
Where do you start fighting an unknown enemy?
The Healthy Trees Healthy Future programme was set up in 2013 to fight RNC, kauri dieback caused by P. agathadicida, and P. cactorum, which is responsible for collar and crown rot in apples. Scion’s Dr Nari Williams is leading the research. Scion is working with other Crown Research Institutes, universities and the forestry industry to investigate ways to control the diseases, breed resistant trees, and prepare future responses.
How infection occurs
The first part of the fight against Phytophthora began with identifying how the pathogen spreads and the infection process. It was discovered that RNC’s zoospores were carried in water droplets with infection driven by misty and rainy conditions. The above average rainfall from winter 2016 and throughout 2017 has been perfect for the pathogen, explaining the widespread appearance of disease this year.
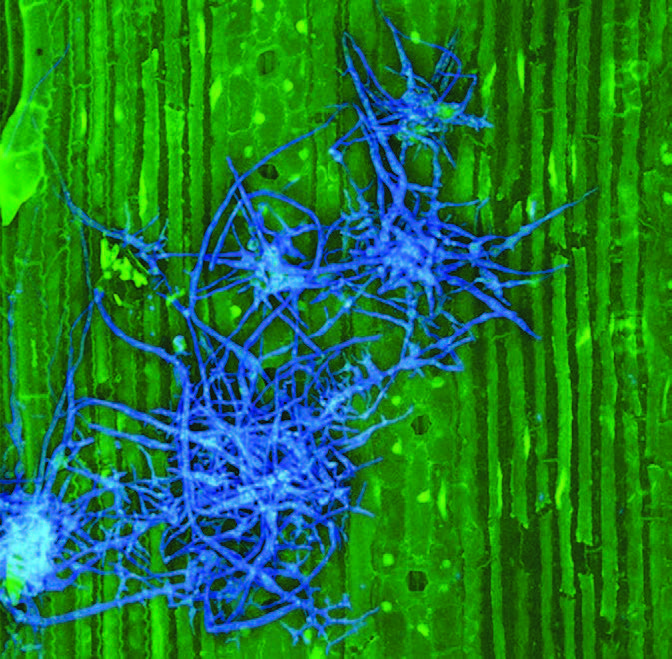
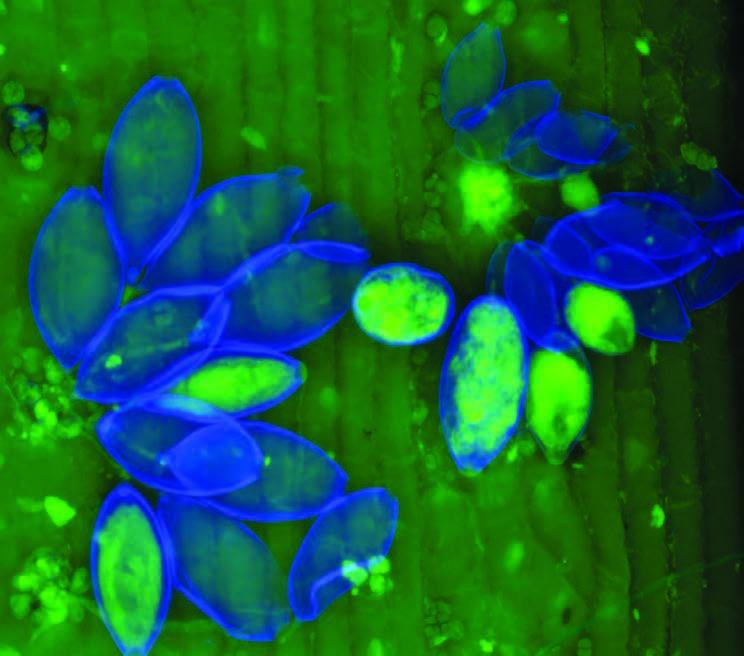
RNC spores landing on a pine needle germinate and their mycelia enter the needle via its pores. Inside the needle they occupy the spaces between cells, not the cells themselves. The infection remains isolated in the needles because a tough lignified layer of cells prevents its access to the rest of the tree. Sporangia later emerge from the stomata to release new zoospores to spread the infection.
Looking for natural resistance
Finding or breeding RNC-resistant trees is a practical and environmentally acceptable long-term solution to the problem. Before the forest pathology team at Scion could start to look for trees with natural resistance they had to develop a reliable method to identify resistant trees.
Over the last couple of years, the team has worked out how to grow the pathogen in the lab and come up with ways to infect detached needles and trees in the laboratory and the field. In all, more than a quarter of a million pine needles have been picked, inoculated, incubated and assessed. To look at infection on a tree-scale, a fogging chamber which can expose whole trees to spore-containing mists was built on the Rotorua campus. Both laboratory methods have confirmed early observations that susceptibility to RNC can vary considerably between trees, which bodes well for the selection of more tolerant trees for planting in disease prone areas.
The most recent step has been to take selected trees into the field and leave them under heavily diseased trees to become infected in forest conditions. The researchers monitored the proportion of needles that remained disease-free over an eight-week exposure period. Where 95 per cent of the trees thought to be most susceptible were infected after two months, around 60 per cent of the foliage of the most resistant trees remained disease free. Needles from this trial have been collected for further analysis to help understand the mechanisms of plant resistance and behaviour of the pathogen.
Questions to be answered
A lot of questions around resistance remain to be answered. For example, do resistant trees produce something which inhibits the pathogen that confers resistance for life? Or is it just that the rate of pathogen growth is slowed and the disease will appear later?

Another alternative is that pathogen reproduction is interrupted, which could limit its establishment and prevent an infection turning into an epidemic. Working out which mechanisms are at play is central to identifying resistance which lasts and markers which enable broad resistance.
So far, comparing P. pluvialis on the surface of a disease-resistant and a susceptible radiata pines has shown that, while the pathogen may be present, it behaves quite differently. Spore-releasing sporangia are produced in abundance on the needles of susceptible genotypes, but not on resistant ones. This has considerable implications for disease suppression in the field.
The data on resistant trees is being used by the Radiata Pine Breeding Company who are looking at other traits, along with RNC resistance, before making any recommendations to the forest industry. Resistance to the pathogen in a particular genotype is all well and good, but if it is linked with an undesirable trait, that genotype may not be suitable for commercial development.
Spray to keep RNC away
Finding a cost-effective, Forest Stewardship Council-compliant, chemical control method to manage outbreaks is another important part of the overall programme. Phosphite, which is used for controlling Phytophthora diseases overseas and in New Zealand by the horticultural industry, was one potential candidate for control. Copper fungicides were another possibility. Copper-based sprays have the advantages of being cheaper than phosphite and are already used by the forestry industry to control dothistroma needle blight, meaning application methods and rates are already well established.
Phosphite was very effective against P. pluvialis in initial controlled trials. However, getting it into radiata pines via the foliage in consistent amounts in the field has proved difficult. A lot of work has been carried out trying to improve fungicide uptake using adjuvants – the chemicals often added to sprays to give even spray coverage, help the spray stick and to penetrate the foliage – but the problem remains.
Copper sprays have also been found to be effective. A major aerial spraying field trial took place in February 2017 comparing phosphite and copper as management methods. One month after application, needles treated with copper showed significantly less disease compared with the control and the phosphite treatment. The best time to apply the copper treatment for RNC seems to be during late summer. The next steps are determining the optimum dosage of copper and the best time to spray.
Gene genie
RNC was first reported in New Zealand about 10 years ago. Its relatively recent arrival was confirmed by taking a close look at its genome. A comparison of the genome of ‘our’ pathogen with that of P. pluvialis from different sources can tell a molecular biologist a lot about the origin of the pathogen found in New Zealand and how long it has been here.
The genomes of all organisms change as mutations take place. As populations drift apart they develop their own characteristics which distinguish them from other populations. Given enough time and separation, new species can evolve. With a good idea of the rate at which mutations occur, scientists can look at gene changes to establish how closely two populations are related and how long they might have been separated from their nearest cousins.
The family tree of New Zealand’s P. pluvialis, as it stands at the moment, shows two different strains have arrived and are most closely related to strains from Oregon. Working backwards from reports of unusual needle disease outbreaks, and taking into account the introduction of stronger import regulations around 2000, Nari Williams has speculated that the pathogen arrived sometime before then, possibly on second-hand equipment or with live plant imports before the tightening of biosecurity regulations.
Douglas-fir is the primary host of P. pluvialis in Oregon. Douglas-fir in New Zealand is also susceptible to the pathogen. Infections tend to go unnoticed as needles are cast but do not change colour and would be more aptly called green needle cast in Douglas-fir. Work is currently underway looking at how widespread the infection is on Douglas- fir in New Zealand.
Chemical fingerprinting
Just as a doctor can diagnose what might be ailing us from the chemicals in our blood and urine, the same thing can be done for plants. As plants respond to stress and infection the chemicals they produce change. The changes start long before a tree starts to show obvious symptoms of disease. If we could monitor these changes we could use them as a diagnostic tool, especially in kauri, and get more insights into what is happening in susceptible and resistance trees and how to bolster resistance.
Work at Scion is being carried out on kauri using cells cultured from seeds. Starting with untouched material removes any other factors that could influence the responses of trees to their environments such as site, rainfall and other pests. The cultured cells are infected in petri dishes. The chemicals produced are then extracted and analysed. The individual components are separated in the results.
Different chemicals are produced by infected and healthy material but the tricky part is identifying them. A chemical reference library for radiata pine, kauri and apples infected with Phytophthora pathogens does not exist. There are many compounds and each unknown chemical needs to be isolated and identified using methods such as mass spectrometry. Stefan Hill, who is the research leader for advanced chemical characterisation, likens the whole process to first discovering the letters to form the words to write books in the library. Work continues in this area with the first samples taken from infected kauri plants in screening trials underway with collaborators at Landcare Research.
Kauri dieback
Kauri trees with odd symptoms were first reported on Great Barrier Island in the 1970s. The disease has now spread to Auckland, Northland and the Coromandel. In the Waitakere Ranges up to 16 per cent of the kauri are showing symptoms. The pathogen responsible, P. agathadicida, spreads in water within the soil to infect kauri roots. The infection grows rapidly through the root systems eventually starving the tree. To date, there are limited options for managing kauri dieback after it has become established. Given the slow regeneration time of kauri, the disease is a cause of major concern.

The best chance for kauri long-term survival is finding trees with inherent resistance to the disease. Finding resistant strains of kauri is a major focus of the Healthy Trees, Healthy Future research programme. Scion is working with mana whenua groups from Northland, Auckland and the Waikato to screen kauri obtained from within their rohe. Seed was collected in 2016 and 2017 and germinated in the new Phytophthora-free growing facilities in the Scion nursery.
Many unknowns
Around 16,000 kauri seedlings have been successfully raised over the last two years. These will be used to test the susceptibility of kauri siblings from the same mother tree to the disease to identify families with potential dieback resistance. According to Nari Williams, seed collected from a mother tree has either been self-pollinated, or pollinated by a pollen donor somewhere upwind. If the seedlings collected from a single mother tree prove to be resistant, there is either a resistant pollen donor in the area, or the mother is resistant.
Early stages of screening have already shown different responses to infection across different kauri lines. However, there are many unknowns. The team is working to understand what the pathogen does when it infects a seedling. Is resistance due to one gene, a group of genes or something else? The aim is also to establish methods to assess disease susceptibility as trees age and across different environments.

Current efforts to slow the spread of kauri dieback are centred on preventing the spread of the pathogen. People who visit the bush in infected areas are urged to sanitise footwear, equipment, dogs’ paws and anything else which may have come into contact with the soil. In places, boardwalks have been built to protect roots and people are asked to stay on designated paths.
Healthy trees and healthy future
The programme fighting New Zealand’s Phytophthora problems has made significant progress over the last four years. The diverse strands of the work are coming together and we now have a good understanding of the pathogens, the infection processes and disease cycles of the main Phytophthora pathogens affecting New Zealand’s tree species.
We now know the pathogen causing red needle cast in radiata pine is widely spread, is helped by persistent rainfall, and peaks in August and September. We know that spraying with copper in late summer provides around three months of protection from RNC which may be sufficient to break the annual epidemic of disease and prevent economic losses.
Pathogen resistant genotypes have been identified and are likely to be available to forest owners and managers in the coming years. We have insights into what causes susceptibility and resistance to Phytophthora pathogens and are in the process of developing higher throughput options for screening.
The collaboration has drawn in national and international expertise and has allowed us to follow many different lines of enquiry. The results so far, and those anticipated in the final two years of the programme, have led to the development of a suite of new methods to fight existing diseases and respond to new pathogens, keeping all our forests growing healthy and strong.
You can find more information on www.healthytrees.co.nz or contact Nari on Nari.williams@scionresearch.com
Funding
The work is funded by the Ministry of Business, Innovation and Employment, the Forest Growers Levy Trust and the Kauri Dieback Programme.
Nari Williams specialises in pathology, management and systems biology of Phytophthora diseases. Michelle Harnett is the senior communication adviser for Scion.

 Farm Forestry New Zealand
Farm Forestry New Zealand

